Antiviral Properties and Potential of Ginger (Zingiber Officinale) and Its Derivatives: A Systematic Review
DOI:
https://doi.org/10.30736/seaj.v7i2.1205Keywords:
Systematic Review, Virucidal Activity, Antiviral Properties, Ginger Extracts, Zingiber officinaleAbstract
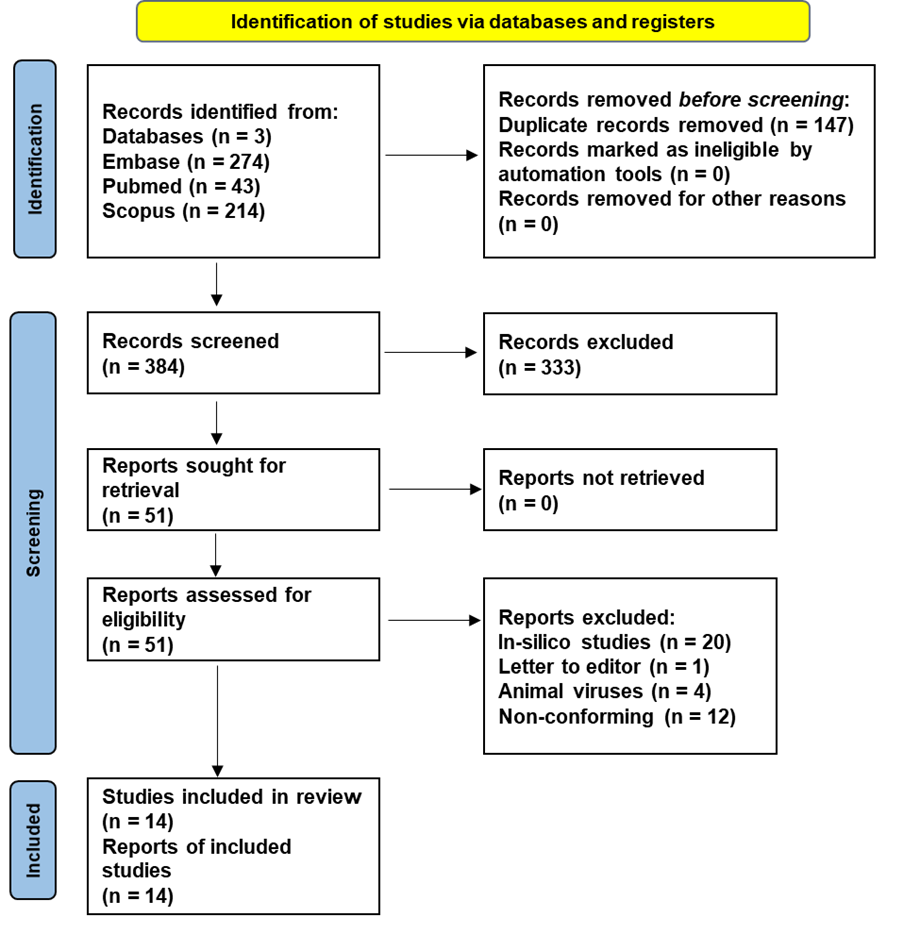
Antiviral Properties and Potential of Ginger (Zingiber Officinale) and Its Derivatives: A Systematic Review. Ginger has long been valued in traditional medicine for its therapeutic benefits. Recently, its antiviral capabilities have attracted significant interest, highlighting its potential as a natural antiviral agent. This systematic review seeks to thoroughly evaluate the antiviral effects of ginger and its active compounds, providing valuable insights to support future research and clinical applications in natural antiviral therapies. A comprehensive electronic search was undertaken across PubMed, Embase, and Scopus databases, employing MeSH terms, Emtree, and relevant synonyms to capture studies on ginger and its antiviral effects. The initial search yielded 531 records, which were de-duplicated and subsequently screened by title and abstract using Rayyan software. Fourteen studies specifically addressing antiviral effects against human pathogens met the inclusion criteria. This systematic review was conducted in strict accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines to ensure rigorous reporting of findings. The majority of included studies were in vitro, revealing anti-viral effects of ginger against various viruses, including Influenza A, Chikungunya, Dengue, hRSV, HSV-2, and SARS-CoV-2 in different cell lines across various concentrations. In addition, Ginger extracts also demonstrated efficacy against Influenza A in both in vivo and in ovo studies, and a randomized controlled trial showcased encouraging antiviral effects targeting SARS-CoV-2. Ginger shows promising antiviral effects in most of the in vitro studies. Translating these findings to in vivo models is imperative for clinical relevance. Further in vivo research is essential before progressing to human studies to ascertain ginger's potential as an effective antiviral agent
Downloads
References
Acosta, P. L., Byrne, A. B., Hijano, D. R., & Talarico, L. B. (2020). Human Type I Interferon Antiviral Effects in Respiratory and Reemerging Viral Infections. Journal of Immunology Research, 2020. https://doi.org/10.1155/2020/1372494
Ahkam, A. H., Hermanto, F. E., Alamsyah, A., Aliyyah, I. H., & Fatchiyah, F. (2020). Virtual prediction of antiviral potential of ginger (Zingiber officinale) bioactive compounds against spike and mpro of SARS-CoV-2 protein. https://doi.org/10.23869/50
Al-Sanea, M. M., Abelyan, N., Abdelgawad, M. A., Musa, A., Ghoneim, M. M., Al-Warhi, T., Aljaeed, N., Alotaibi, O. J., Alnusaire, T. S., Abdelwahab, S. F., Helmy, A., Abdelmohsen, U. R., & Youssif, K. A. (2021). Strawberry and ginger silver nanoparticles as potential inhibitors for SARS-CoV-2, assisted by in silico modeling and metabolic profiling. Antibiotics (Basel), 10(7), 824. https://doi.org/10.3390/antibiotics10070824
Altindis, M., & Kahraman Kilbas, E. P. (2023). Managing Viral Emerging Infectious Diseases via Current and Future Molecular Diagnostics. In Diagnostics (Vol. 13, Issue 8). https://doi.org/10.3390/diagnostics13081421
Ballester, P., Cerdá, B., Arcusa, R., Marhuenda, J., Yamedjeu, K., & Zafrilla, P. (2022). Effect of Ginger on Inflammatory Diseases. Molecules (Basel, Switzerland), 27(21). https://doi.org/10.3390/molecules27217223
Chang, J. S., Wang, K. C., Yeh, C. F., Shieh, D. E., & Chiang, L. C. (2013). Fresh ginger (Zingiber officinale) has anti-viral activity against human respiratory syncytial virus in human respiratory tract cell lines. Journal of Ethnopharmacology, 145(1), 146–151. https://doi.org/10.1016/j.jep.2012.10.043
Denyer, C. V., Jackson, P., Loakes, D. M., Ellis, M. R., & Young, D. A. (1994). Isolation of antirhinoviral sesquiterpenes from ginger (Zingiber officinale). Journal of Natural Products, 57(5), 658–662. https://doi.org/10.1021/np50107a017
Dutta, A., Hsiao, S. H., Hung, C. Y., Chang, C. S., Lin, Y. C., Lin, C. Y., Chen, T. C., & Huang, C. T. (2023). Effect of [6]-gingerol on viral neuraminidase and hemagglutinin-specific T cell immunity in severe influenza. Phytomedicine Plus, 3(1), 0–7. https://doi.org/10.1016/j.phyplu.2022.100387
Edo, G. I., Onoharigho, F. O., Jikah, A. N., Ezekiel, G. O., Essaghah, A. E. A., Ekokotu, H. A., Ugbune, U., Oghroro, E. E. A., Emakpor, O. L., Ainyanbhor, I. E., Akpoghelie, P. O., Ojulari, A. E., Okoronkwo, K. A., & Owheruo, J. O. (2024). Evaluation of the physicochemical, phytochemical, and anti-bacterial potential of Zingiber officinale (ginger). Food Chemistry Advances, 4, 100625. https://doi.org/https://doi.org/10.1016/j.focha.2024.100625
Glitscher, M., Himmelsbach, K., Woytinek, K., Johne, R., Reuter, A., Spiric, J., Schwaben, L., Grünweller, A., & Hildt, E. (2018). Inhibition of hepatitis E virus spread by the natural compound silvestrol. Viruses, 10(6). https://doi.org/10.3390/v10060301
Goncalves, B. C., Lopes Barbosa, M. G., Silva Olak, A. P., Belebecha Terezo, N., Nishi, L., Watanabe, M. A., Marinello, P., Zendrini Rechenchoski, D., Dejato Rocha, S. P., & Faccin-Galhardi, L. C. (2021). Antiviral therapies: advances and perspectives. Fundamental & Clinical Pharmacology, 35(2), 305–320. https://doi.org/10.1111/fcp.12609
Hasan, T. N., Naqvi, S. S., Rehman, M. U., Ullah, R., Ammad, M., Arshad, N., Ain, Q. U., Perween, S., & Hussain, A. (2023). Ginger ring compounds as an inhibitor of spike binding protein of alpha, beta, gamma, and delta variants of SARS-CoV-2: An in-silico study. Narra J, 3(1), e98. https://doi.org/10.52225/narra.v3i1.98
Hayati, R. F., Better, C. D., Denis, D., Komarudin, A. G., Bowolaksono, A., Yohan, B., & Sasmono, R. T. (2021). [6]-Gingerol inhibits chikungunya virus infection by suppressing viral replication. BioMed Research International, 2021. https://doi.org/10.1155/2021/6623400
Imanishi, N., Andoh, T., Mantani, N., Sakai, S., Terasawa, K., Shimada, Y., Sato, M., Katada, Y., Ueda, K., & Ochiai, H. (2006). Macrophage-mediated inhibitory effect of Zingiber officinale Rosc, a traditional Oriental herbal medicine, on the growth of influenza A/Aichi/2/68 virus. American Journal of Chinese Medicine, 34(1), 157–169. https://doi.org/10.1142/S0192415X06003722
Johnson, J. B., Mani, J. S., White, S., Brown, P., & Naiker, M. (2021). Quantitative profiling of gingerol and its derivatives in Australian ginger. Journal of Food Composition and Analysis, 104, 104190. https://doi.org/https://doi.org/10.1016/j.jfca.2021.104190
José-Rita, B. J., Bertin, G. K., Ibrahime, S. K., Yannick, K., Erick-Kévin, B. G., Riphin, K. L., Ceylan, R., David, N. J., Zengin, G., & Mireille, D. (2022). Study of the chemical and in vitro cytotoxic activities of essential oils (EOs) of two plants from the Ivorian flora (Lippia multiflora and Zingiber officinale) and their antiviral activities against non-enveloped viruses. South African Journal of Botany, 151, 387–393. https://doi.org/10.1016/j.sajb.2022.03.053
Kamankesh, F., Ganji, A., Ghazavi, A., & Mosayebi, G. (2023). The Anti-Inflammatory Effect of Ginger Extract on the Animal Model of Multiple Sclerosis. Iranian Journal of Immunology : IJI, 20(2), 211–218. https://doi.org/10.22034/iji.2023.97156.2482
Kaushik, S., Jangra, G., Kundu, V., Yadav, J. P., & Kaushik, S. (2020). Anti-viral activity of Zingiber officinale (Ginger) ingredients against the Chikungunya virus. VirusDisease, 31(3), 270–276. https://doi.org/10.1007/s13337-020-00584-0
Kawaoka, Y. (2023). Addressing the Threat of Emerging Viral Infections. The Keio Journal of Medicine, 72(1), 27. https://doi.org/10.2302/kjm.ABSTRACT_72_1-2
Kharisma, V. D., Utami, S. L., Rizky, W. C., Dings, T. G. A., Ullah, M. E., Jakhmola, V., & Nugraha, A. P. (2023). Molecular docking study of Zingiber officinale Roscoe compounds as a mumps virus nucleoprotein inhibitor. Dent. J., 56(1), 23–29. https://doi.org/10.20473/j.djmkg.v56.i1.p23-29
Koch, C., Reichling, J., Schneele, J., & Schnitzler, P. (2008). Inhibitory effect of essential oils against herpes simplex virus type 2. Phytomedicine, 15(1–2), 71–78. https://doi.org/10.1016/j.phymed.2007.09.003
Leka, K., Hamann, C., Desdemoustier, P., Frédérich, M., Garigliany, M. M., & Ledoux, A. (2022). In vitro antiviral activity against SARS-CoV-2 of common herbal medicinal extracts and their bioactive compounds. Phytotherapy Research, 36(8), 3013–3015. https://doi.org/10.1002/ptr.7463
Li Jian, Zhang Jia, & Zhou Litao. (2022). Effects of Ginger on Clinical Features and Disease Severity of Patients With Severe Acute Respiratory Syndrome Due To Covid-19: a Randomized Controlled Trial Study. Acta Medica Mediterranea, 38(625), 625–630. https://doi.org/10.19193/0393-6384
Mehyar, N. (2023). Coronaviruses SARS-CoV, MERS-CoV, and SARS-CoV-2 helicase inhibitors: a systematic review of in vitro studies. Journal of Virus Eradication, 9(2). https://doi.org/10.1016/j.jve.2023.100327
Mukherjee, S., Weiner, W. S., Schroeder, C. E., Simpson, D. S., Hanson, A. M., Sweeney, N. L., Marvin, R. K., Ndjomou, J., Kolli, R., Isailovic, D., Schoenen, F. J., & Frick, D. N. (2014). Ebselen inhibits hepatitis C virus NS3 helicase binding to nucleic acid and prevents viral replication. ACS Chemical Biology, 9(10), 2393–2403. https://doi.org/10.1021/cb500512z
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Nouman Ahmad, Hamdan Ahmad, Dewi Syarifah, Vivian Soetikno

This work is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.
Authors who publish with this journal agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution-ShareAlike 4.0 International License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial publication in this journal.
- Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See The Effect of Open Access).

This work is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.








